
The Unit is involved in all the computational work (modeling and simulation) needed during the stages of discovery-development and application of pharmaceutical products into clinical practice.
More specifically, the computational work covers:
-
computational methodologies at the molecular level (e.g., drug – receptor interaction) which are required when searching for a new active substance,
-
quantitative structure relationships between molecular properties (e.g., MW, surface area) or physicochemical characteristics (e.g., solubility) and drug action or transit through the body (absorption, distribution, metabolism) characteristics,
-
computational design for development of the drug dosage form e.g., set specific release or dissolution properties, and the development of in vitro-in vivo correlations (IVIVC),
-
computational methods in pharmacokinetics and pharmacodynamics using non-compartmental or compartmental methods, as well as population approaches relying on nonlinear mixed effect models,
-
the mathematical and statistical analysis for the design and assessment of clinical and bioequivalence studies,
-
the application of population techniques based either on Bayesian approximations or control theory for determination, for setting the appropriate dosage regimen for narrow therapeutic index drugs,
-
all kinds of meta-analyses regarding drug action, side-effects, interactions, etc., in clinical use of drugs,
-
the use of bio-informatics in the drug design of biotechnology drugs.
In summary, the Unit deals with the following six subject areas:
-
Informatics for Drug Discovery / Chemoinformatics
-
Informatics for Quality-by-Design and Formulation Development
-
Informatics for Biopharmaceutics, Pharmacokinetics, and Pharmacodynamics
-
Informatics for Clinical Trials and Clinical Practice
-
Informatics for Meta-Analysis
-
Applications of Bioinformatics to drug design-action of biotechnology drugs.
A diagram of the subject areas in which the ‘PharmaInformatics’ Unit is involved is shown in Fig. 1.
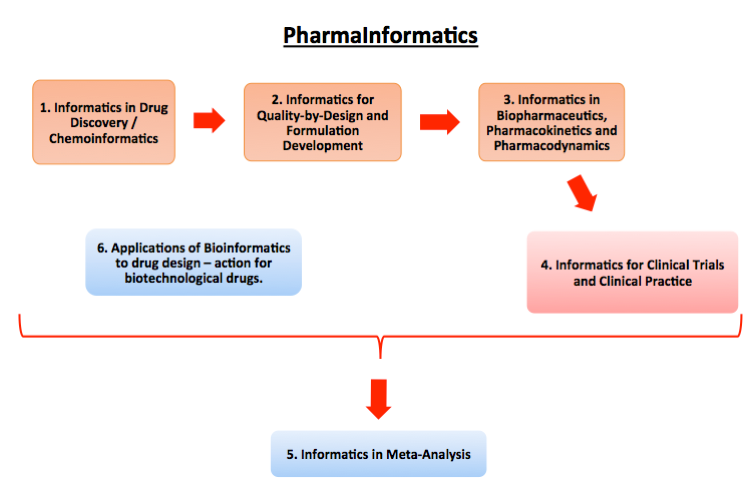
Figure 1. A schematic representation of the subject areas in which the PharmaInformatics Unit is involved.
Scientific Team
Head of the Unit
-
Panos Macheras: Professor Emeritus, Dpt. of Pharmacy, National and Kapodistrian University of Athens, Greece.
Members:
-
Dimitrios Hatziavramidis: Professor, School of Chemical Engineering, National Technical University of Athens, Greece.
-
Emmanuel Mikros: Professor, Dpt. of Pharmacy, National and Kapodistrian University of Athens, Greece.
-
Haralambos Sarimveis: Professor, School of Chemical Engineering, National Technical University of Athens, Greece.
-
Dimitrios Rekkas: Assoc. Professor, Dpt. of Pharmacy, National and Kapodistrian University of Athens, Greece.
-
Aris Dokoumetzidis: Assistant Professor, Dpt. of Pharmacy, National and Kapodistrian University of Athens, Greece.
-
Evangelos Evangelou: Assistant Professor, Clinical and Molecular Epidemiology at University of Ioannina, Greece.
-
Kosmas Kosmidis: Dpt of Physics, Aristotle University of Thessaloniki, Greece
-
Ioannis Vizirianakis Associate Professor, Aristotle University of Thessaloniki, Greece
-
Maria Gazouli, Associate Professor, School of Medicine, National and Kapodistrian University of Athens
-
Dimitris Goussis, Professor, School of Applied Mathematics and Physical Sciences, National Technical University of Athens, Greece
-
Despina Sanoudou, Assistant Professor, School of Medicine, National and Kapodistrian University of Athens, Greece
-
Athanassios Iliadis, Professor Emeritus, School of Pharmacy, University of Marseilles, France
-
Theodoros Christopoulos, Professor, Dpt. of Chemistry, University of Patras, Greece
-
Ioannis Boletis, Professor, School of Medicine, National and Kapodistrian University of Athens, Greece